Applicant & Spokesperson
Prof. Dr. Susanne Grässel
University Hospital Regensburg
Orthopedic Surgery
Experimental Orthopedics,
ZMB - Biopark I
Am Biopark 9
93053 Regensburg
Phone: +49 (0) 941 943 5065
Email: susanne.graessel@ukr.de
Role of the sensory nervous system in subchondral bone remodeling in OA pathology
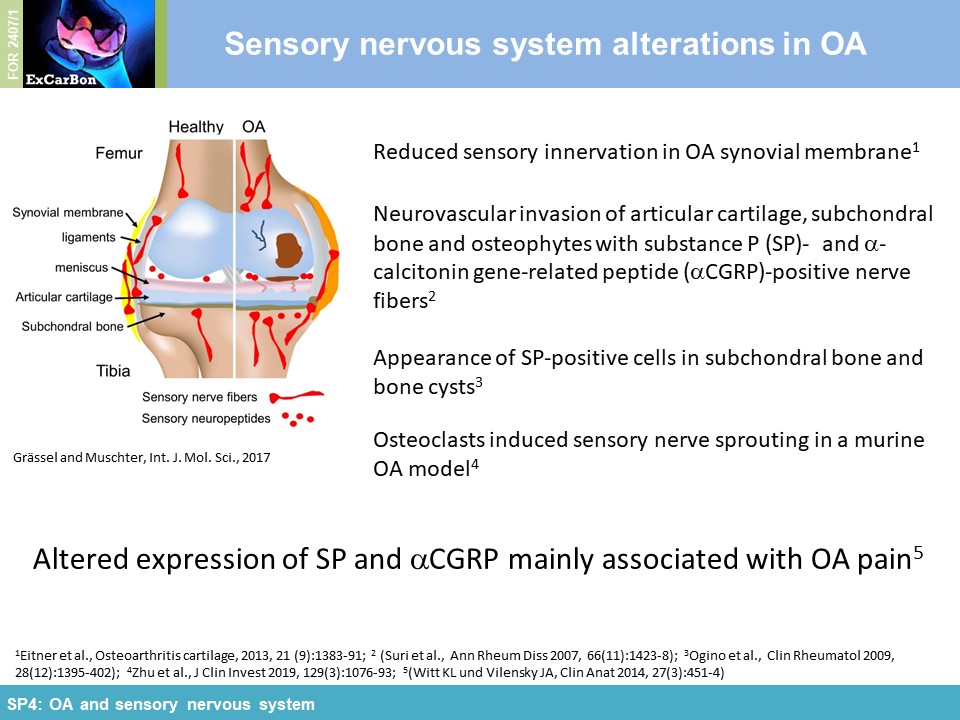
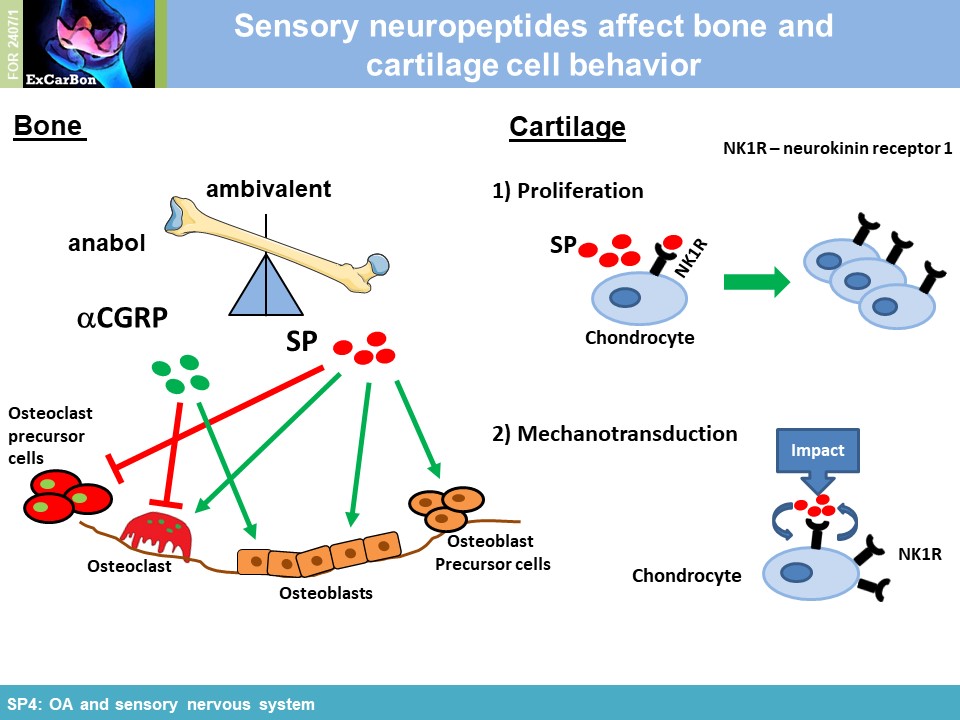
The joints are innervated by calcitonin gene-related peptide (aCGRP)- and substance P (SP) positive sensory nerve fibers and there is accumulating evidence that sensory neurotransmitters have crucial trophic effects which are essential for proper bone metabolism and bone remodeling. Alteration of sensory joint innervation might be partly responsible for degenerative changes which contribute to development of osteoarthritis. Underlying molecular mechanisms which contribute to abnormal subchondral bone remodeling and osteophyte formation during the pathogenesis of OA due to changes in sensory joint innervation and their respective neurotransmitter milieu are mostly unknown. We hypothesize that the profile of sensory nerve fibers in subchondral bone is altered already early in OA and this alteration affects osteoclast number/activity during OA progression. We propose that inhibition of osteoclasts improves subchondral bone pathophysiology and ameliorate OA pathogenesis (WP1). Likely, sensory neurotransmitters modulate joint repair processes in response to aberrant mechanical loading (WP2) and thus mechanical stress may modulate neurotransmitter effects on osteoclast/BMM metabolism in cell culture (WP3). We further assume that the profile of neurotransmitter-receptor (neurokinin-1- receptor and CGRP-receptor) regulatory miRs is altered in synovial- and bone marrow derived macrophages during pathogenesis of OA (WP4).
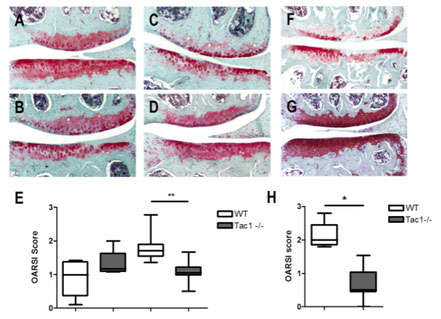
We did not observe differences in spontaneous OA-scoring in the 12 month old group (A, B, E), however after 16 months WT-mice develop significant more severe spontaneous OA scores compared to Tac1-deficient mice (C, D, E). 4 weeks after induction of OA by DMM, WT mice obtained a significant higher OA-score compared to Tac1-deficient mice (F, G, H). 12 months old group N=6, 16 months old group N=8, 4 weeks after DMM group N=7, bars = 200 µm.

Top: Immunofluorescence staining of osteoclast/BMM cultures for NK1R or CGRP-receptor (red).Cell nuclei are stained with DAPI (blue). Arrows indicate multi-nuclear
osteoclasts. Bottom: Respective light microscopic images. Magnification for all images = 400x.
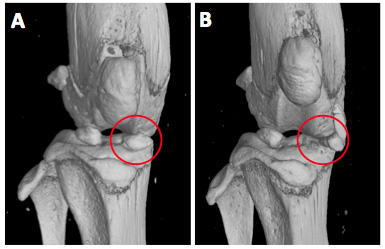
A) µCT analysis of sham-operated knees from C57BL/6J mice shows normal localization of the medial meniscus (red circle) in the
weight bearing region between femoral condyle and tibial plateau. B) Transection of the medial meniscotibial ligament provokes translocation of the medial meniscus (red circle)
leading to instability and progressive osteoarthritis in mice.
Objectives and Work Program for the Second Funding Period
Key Achievements of the First Funding Period
The emerging consensus that OA is essentially the cumulative failure of the composing tissues of the whole diarthrodial joint directed the research of ExCarBon to
explore the time-dependent changes in the subchondral bone (SB) during the onset and progression of OA. In Cluster II (SP4 and SP5), we aimed to identify structural, molecular and genetic factors that regulate SB and impact on OA pathogenesis. Within the Research Unit, we have investigated the
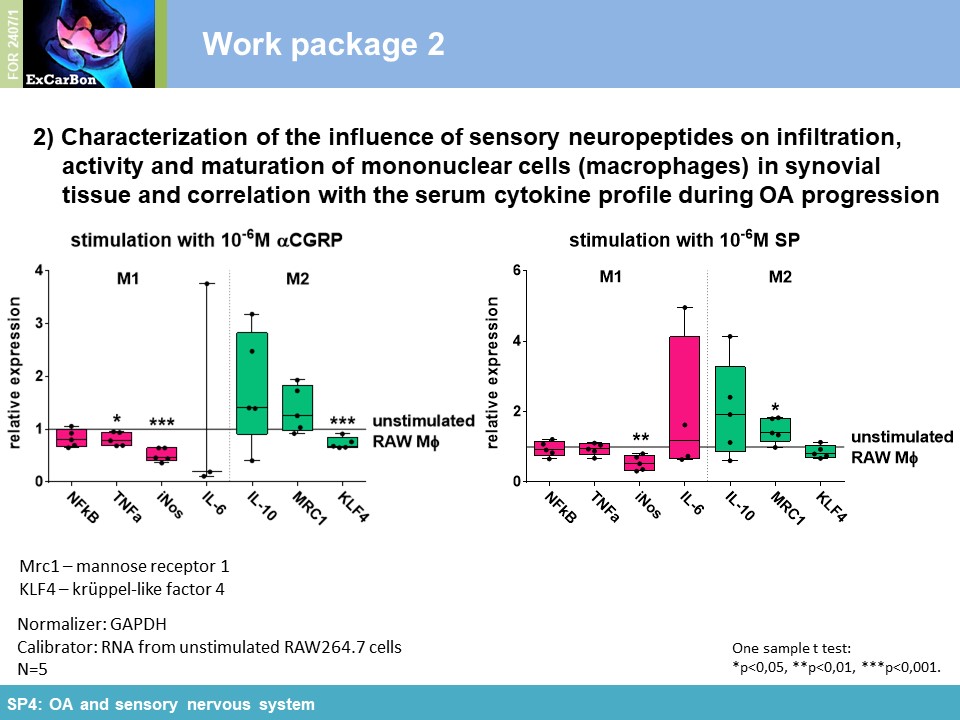
impact of substance P (SP) and alpha calcitonin gene-related peptide (αCGRP), two neuropeptides released by sensory nerves innervating SB, on pathophysiology of the joint (SP4). In order to
elucidate the impact of these sensory neuropeptides on SB and cartilage during joint degradation, we have surgically induced OA via DMM in mice either deficient for SP (tachykinin 1-/-; Tac1-/-)
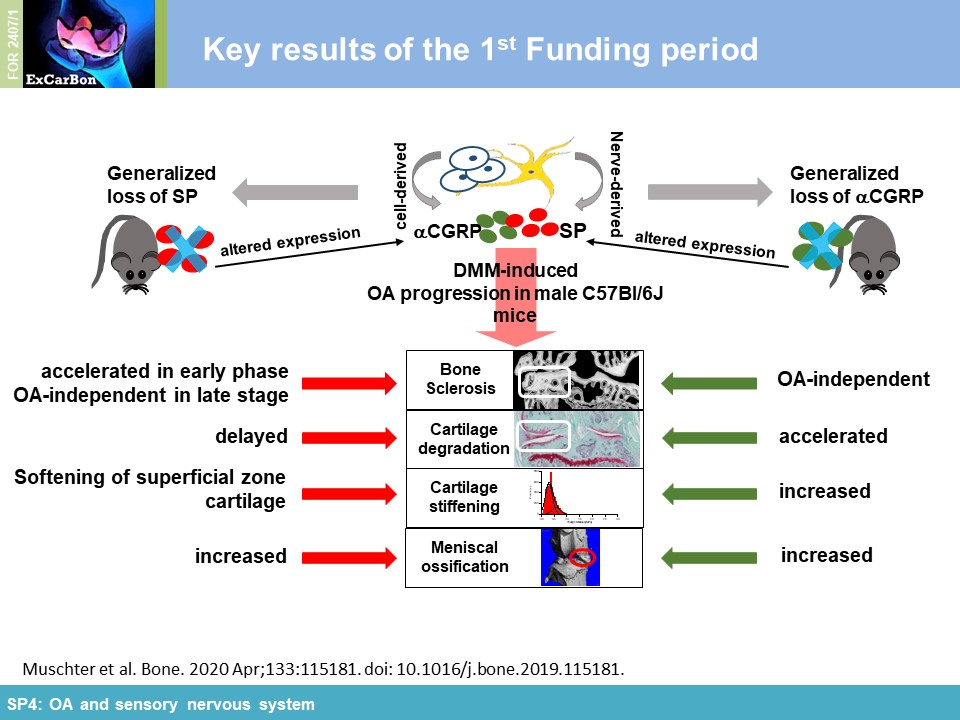
or αCGRP (αCGRP-/-). Cartilage matrix degradation was accelerated in both neuropeptide-deficient mouse strains in the medial femur condyle (Figure 1A) which was accompanied by
altered biomechanical properties (increased stiffness) of the articular cartilage assessed by indentation-type AFM (IT-AFM) compared to WT mice (Figure 1B) (together with
SP1). Applying µCT and high resolution nanoCT (with SP5), we
found clear evidence that both neuropeptides protect from DMM-induced subchondral bone degeneration/sclerosis and ectopic bone formation affecting bone microarchitecture already in an early OA
stage (Figure 1C). We assessed serum concentrations of SP and aCGRP in WT mice over OA progression as potential biomarkers for determining early OA onset. Both sensory
neuropeptides and their receptors are involved in murine macrophage mechanotransduction affecting neuropeptide impact on adhesion and ROS activity [1] . Mechanical cyclic stretch induced
upregulation of NK1R and CRLR gene expression (Figure 2A) and reduced SP gene and protein expression (Figure 2A-B). NK1R protein expression decreased in
stretched RAW264.7 macrophages compared to non-stretched cells, whereas CRLR protein expression was increased after 1 and 3 days of stretching (Figure 2C). Figure
3 summarizes the influence of cyclic stretch on RAW264.7 macrophage cells in the context of sensory neuropeptide stimulation. Taken together, our results imply that both SP and αCGRP
mostly have preserving functions for bone and cartilage in the context of structural and mechanical properties.
[1] Muschter D, Beiderbeck AS, Späth T, Kirschneck C, Schröder A, Grässel S. Sensory Neuropeptides and their Receptors Participate in Mechano-Regulation of Murine Macrophages. Int J Mol Sci. 2019 Jan 24;20(3):503. doi: 10.3390/ijms20030503.
Figure 1. A) Alterations in cartilage appearance of the medial femoral condyle (MFC) and the medial tibia plateau (MTP) was evaluated in Safranin O-stained frontal knee joint sections according to the OARSI guidelines. OARSI score of the MFC was significantly increased as early as 4 weeks after OA induction in neuropeptide-deficient mice, but not in WT mice. MTP cartilage showed signs of degradation in all genotypes at this time-point. After 8 weeks, MFC score of DMM groups was highly variable and only WT DMM mice score was significantly enhanced compared to Sham WT mice. The MTP score was increased in all DMM groups compared to the respective Sham group (N=6). B) Measurement of cartilage stiffness in the middle zone 8 weeks after DMM and Sham surgery using Indentation-type atomic force (IT-AFM). Cartilage stiffness of WT mice was similar in DMM and Sham mice. In aCGRP-/- and Tac1-/-, OA induction caused a significant increase in cartilage stiffness in the middle zone after 8 weeks (N=3). C) NanoCT analysis of the meniscle ossicles that developed in the medial meniscus 8 weeks after OA induction. All DMM groups revealed a profound higher bone surface equalling a larger size of the ossicles compared to Sham. Bone surface of the ossicles in the neuropeptide-deficient mice was higher compared to WT, especially in the aCGRP-/- mice (N=3).
Figure 2. A) Mechanical cyclic stretch of murine RAW264.7 macrophages induced an upregulation of mRNA for the neurokinin receptor 1 (NK1R) and the receptor subunit calcitonin receptor-like receptor (CRLR) of the aCGRP receptor. mRNA expression of the SP was reduced by cyclic stretch. B) Release of SP by RAW264.7 macrophages into the supernatant was measured after 1, 2 and 3 days of cyclic stretch. We observed a significant reduction of SP in the cell culture supernatant after 1 day of mechanical stress that subsided when the stretch was extended to 2 or 3 days. C) Receptor protein expression of NK1R and CRLR after cyclic stretch was analyzed by Western blot. NK1R expression decreased in stretched RAW264.7 macrophages compared to non-stretched cells, whereas CRLR expression was increased after 1 and 3 days of stretching.
Figure 3. Schematic summary depicting the influence of cyclic stretch on RAW264.7 macrophage cells in the context of sensory neuropeptide stimulation. I) Physiological stimulation with SP and aCGRP decrease mRNA expression of their respective receptors, NK1R and Ramp1 (aCGRP-specific subunit of the aCGRP receptor) in unloaded conditions. Cyclic stretch induced upregulation of mRNA for CRLR (unspecific aCGRP receptor subunit) and NK1R. aCGRP further upregulated CRLR gene expression resulting in increased CRLR protein expression. Contrary, albeit upregulation of NK1R mRNA; protein expression decreased, possibly by reduction of receptor ligand SP that was also decreased by cyclic stretch. In loaded RAW264.7 cells, cyclic stretch might induce a negative feedback loop involving the downregulation of the neuropeptide SP and its respective receptor, NK1R. II) Comparison of cellular traits like adhesion, proliferation, apoptosis, ROS activity and the macrophage phenotype in loaded RAW264.7 macrophages to unloaded controls demonstrated a strong induction of apoptosis and a phenotypic change towards the M1 phenotype. In unloaded cells, SP inhibited adhesion, increased ROS activity and induced expression of M2 macrophage marker genes. aCGRP effects were marginal. Oppositely, cyclic stretch sensitized macrophages to strong inhibition of adhesion by SP as well as a strong increase in cellular ROS activity by stimulation with SP and aCGRP. 1The same increase of apoptosis after loading was observed in bone marrow-derived macrophages from mice that underwent surgical OA induction.
aCGRP – alpha calcitonin gene-related peptide, CRLR – calcitonin receptor-like receptor, NK1R - neurokinin receptor 1, Ramp1 – receptor activity modifying protein 1, SP – substance P.